Antihypertensive
medications and hepatocellular carcinoma risk: a systematic review
Shadman
Newaz 1*, Fahmida Zaman 1, Ayesha Noor 2, Promita
Das 3, Fariha Tanjim 1, Farhana Ferdaus Raisa 1,
Muhsina Farhat Lubaba 1, Jannat Ara Tina 1, Supritom
Sarker1
1 Tangail
Medical College, Tangail, Bangladesh
2 Department
of Pharmacy, Jahangirnagar University, Dhaka, Bangladesh
3 Dinajpur
Medical College, Dinajpur, Bangladesh
Corresponding Author: Shadman
Newaz
* Email: shadmannewaz11@gmail.com
Abstract
Introduction: Emerging evidence
suggests that antihypertensive medications may influence the risk, progression,
and survival outcomes of hepatocellular carcinoma (HCC). However, findings
across studies remain inconsistent. This systematic review aims to evaluate and
synthesize current data on the associations between different classes of
antihypertensive drugs and liver cancer outcomes.
Materials and methods: A systematic review was conducted, incorporating randomized controlled
trials, cohort studies, retrospective analyses, and in vitro studies that
investigated the relationship between antihypertensive medications and HCC.
Extracted data included study design, population characteristics, drug
categories, primary outcomes, and study limitations.
Results: Nine studies met the inclusion criteria, encompassing diverse study
designs and patient populations. Renin-angiotensin system (RAS)
inhibitors—including ACE inhibitors and angiotensin receptor blockers
(ARBs)—were most consistently associated with reduced HCC incidence and
improved survival. Thiazide diuretics demonstrated potential protective effects
in genetic studies, though results were mixed in larger population-based
analyses. Beta-blockers yielded inconclusive evidence: while some studies
linked them to increased HCC risk, others found neutral or beneficial effects,
particularly for non-selective Beta-blockers in patients with established HCC.
Additionally, one preclinical study highlighted possible anti-cancer activity
of agents like chlorpromazine and prazosin.
Conclusion: RAS inhibitors show the strongest and most consistent evidence for a
protective effect against HCC development and progression among
antihypertensive drug classes. Certain non-selective Beta-blockers may also
offer survival benefits in specific patient populations. However, conflicting
findings and methodological limitations across studies underscore the need for
high-quality prospective research to confirm these associations and inform
clinical practice
Keywords: Hepatocellular carcinoma, Antihypertensive drug, ACE inhibitors, ARBs,
Beta-blockers, Diuretics, Liver cancer
Introduction
Hypertension is a widespread global health
concern and a primary contributor to cardiovascular disease (1–4). Globally, approximately
1.28 billion adults aged 30 to 79 are affected by hypertension (5), which is recognized as the
leading risk factor for mortality worldwide (5,6). In parallel, liver cancer
continues to pose a major global health challenge. As of 2024, it ranks as the
sixth most common cancer and the fourth leading cause of cancer-related deaths,
accounting for nearly 800,000 fatalities each year. Hepatocellular carcinoma
(HCC) constitutes about 90% of all primary liver cancers (7,8).
Most individuals diagnosed with stage 1
hypertension or higher are treated with antihypertensive medications (9–11). Common
first-line agents include Angiotensin-Converting Enzyme Inhibitors (ACEIs),
Angiotensin II Receptor Blockers (ARBs), Calcium Channel Blockers (CCBs), and
thiazide diuretics. Other medications such as Beta-blockers, loop diuretics,
vasodilators, and α-blockers are typically used as second-line treatments
in particular clinical contexts (12,13). Research
exploring the relationship between antihypertensive therapies and cancer risk
has produced inconsistent findings. These variations may be influenced by
geographic location, preexisting conditions, concurrent comorbidities, and
unknown underlying mechanisms.
Specifically, the connection between
antihypertensive drug use and liver cancer has not been extensively studied,
and current data do not establish a definitive causal link (14–16). Nevertheless,
the relationship is believed to be multifactorial and remains under
investigation. Some studies suggest that certain antihypertensive drug classes
may influence tumor development (17–20). The
Renin-Angiotensin-Aldosterone System (RAAS), for instance, has been implicated
in cancer biology beyond its role in blood pressure regulation. It is
hypothesized that modulation of Angiotensin II activity by drugs like ACEIs and
ARBs could affect angiogenesis (21–24) and thereby
influence liver tumorigenesis.
ACEIs and ARBs have been associated with
reduced cancer incidence and improved survival in several observational studies
(25–27). Moreover,
Beta-blockers, thiazide diuretics, and some CCBs have demonstrated
anti-angiogenic properties, which could influence tumor growth (27,28). Conversely,
other research—such as one case-control study—reported a slight increase in
colorectal cancer risk with ACEI and ARB use, raising concerns about potential
implications for liver metastasis (29).
Given the conflicting data and the absence of
definitive conclusions, a significant research gap persists regarding the role
of blood pressure medications in HCC development (25,27,28,30). The potential
interactions between antihypertensive agents and liver cancer remain
underexplored (31,32). This review
seeks to evaluate both the direct and indirect roles of these medications in
HCC, examine the conflicting effects of ACEIs and ARBs and their clinical
implications, address gaps in current knowledge, and highlight new directions
for future research aimed at clarifying these associations.
Materials and methods
Study Design
and Protocol Registration
This systematic review was guided by a pre-established protocol
registered on the Open Science Framework. The review process adhered strictly
to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses
(PRISMA) guidelines to ensure transparent and rigorous reporting.
Eligibility
Criteria
Studies published between 1st January 2015 and 28th
February 2025 were eligible for inclusion if they explored the association
between antihypertensive medications and liver cancer-related outcomes.
Included study designs comprised randomized controlled trials, cohort and
case-control studies, and observational research. Only articles published in
English were considered. To be included, studies had to focus on individuals
with hypertension and assess the effects of antihypertensive drugs on liver
cancer risk, progression, or mortality. Studies were excluded if they were
non-English, lacked extractable data, were protocols only, or discussed other
cancer types without clear relevance to liver cancer in hypertensive patients.
Research published before 2015 was also excluded.
Search Strategy
An extensive literature search was conducted using four prominent
electronic databases: PubMed, ScienceDirect, the Cochrane Central Register of
Controlled Trials (CENTRAL), and Mendeley. The search employed a mix of Medical
Subject Headings (MeSH) and keyword-based queries focused on antihypertensive
drugs, liver cancer, and hypertension.
Key search terms included:
·
For drug
types: "ACE inhibitors" OR "angiotensin-converting enzyme
inhibitors" OR "ARBs" OR "angiotensin II receptor
blockers" OR "beta-blockers" OR "calcium channel
blockers" OR "diuretics" OR "renin-angiotensin system"
OR "antihypertensive agents"
·
For
cancer: "liver cancer" OR "hepatocellular carcinoma" OR
"liver carcinoma" OR "hepatic neoplasms"
·
Subtypes:
"hepatocellular carcinoma" OR "cholangiocarcinoma" OR
"liver metastasis"
The search was broadened using additional terms such as:
·
"liver cancer incidence," "progression,"
"recurrence," "mortality," and "survival"
·
Combined
queries like "hypertension treatment" OR "cardiovascular
drugs" AND "liver cancer risk," and "antihypertensive side
effects" AND "liver cancer survival"
Reference lists from key studies and relevant reviews were manually
screened to capture any overlooked studies. The initial database search was
conducted on January 26, 2025, and updated on February 26, 2025.
Screening and
Data Extraction
The screening process was conducted using Rayyan software to facilitate
duplicate removal and streamline the title/abstract screening phase. Two
independent reviewers (JT and FT) conducted the initial screening, and any
disagreements were resolved through consultation with a third reviewer (SS).
Full-text articles of potentially eligible studies were retrieved and reviewed
for final inclusion.
Data extraction was carried out using a structured Excel template, which
collected information on study design, sample population, drug class, liver
cancer outcomes, and key findings. Extraction was primarily handled by SN, with
independent verification of 50% of the data entries by FZ and PD for quality
assurance.
Quality
Assessment
Although the review primarily aimed to summarize the breadth of available
evidence rather than perform a critical quality assessment, potential
limitations and sources of bias in each study were noted descriptively. Where
applicable, formal tools such as the Newcastle-Ottawa Scale were used to
appraise the quality of cohort and case-control studies. No studies were
excluded based on quality scores alone.
Data Synthesis
Due to substantial heterogeneity in the
included studies—across study design, sample demographics, drug classification,
outcome measurement, and follow-up duration—a meta-analytic approach was not
feasible. While formal statistical heterogeneity (e.g., I²) could not be
calculated, qualitative indicators of variability such as differing comparator
groups, inconsistent effect directionality, and variation in drug dosage/timing
were noted across studies.
As a result, findings were synthesized narratively to provide a comprehensive
overview of the relationships between different classes of antihypertensive
medications and liver cancer outcomes. This approach allowed for thematic
comparison across diverse methodologies and helped contextualize apparent
contradictions in the literature.
Bias Assessment
To evaluate potential biases in the included studies, recognized
assessment frameworks were used. The Cochrane Risk of Bias tool was applied to
analyze aspects such as selection, performance, detection, and reporting bias.
Each study underwent independent review by multiple researchers to enhance
objectivity and consistency. This process aimed to thoroughly explore and
report the possible biases affecting study outcomes and to support the
credibility of the review's conclusions.
Results
The selection process for this systematic
review, as outlined in the PRISMA flow diagram (Figure 1), aimed to determine
the potential effects of antihypertensive drugs on liver cancer outcomes. A
total of 1,376 records were retrieved from three databases: PubMed (10),
ScienceDirect (1,000), and Mendeley (366). After removing 66 duplicates, 1,310
records were screened by title and abstract. Following this, 1,299 records were
excluded due to irrelevance or not meeting the inclusion criteria. 11 full-text
articles were reviewed, with 2 subsequently excluded for being unrelated,
leaving 9 studies that met the eligibility criteria and were included in the
final review. This systematic selection approach promotes transparency and
methodological rigor (Figure 1).
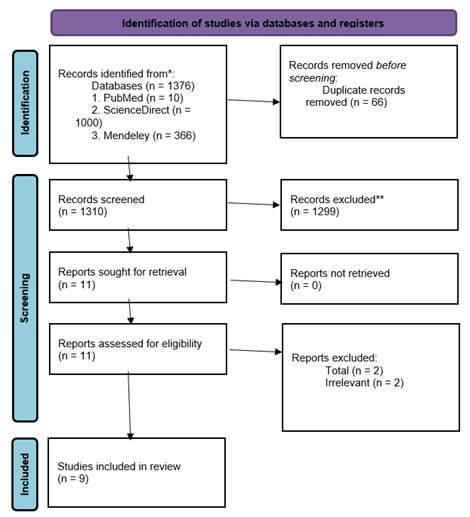
Figure 1. Prisma flow diagram illustrating the study selection process. The
flow diagram outlines the systematic selection of studies included in this
review. A total of 1,376 records were identified through database searches
(PubMed: 10, ScienceDirect: 1,000, Mendeley: 366). After removing 66
duplicates, 1,310 records were screened by title and abstract. Of these, 1,299
were excluded for not meeting the inclusion criteria. Eleven full-text articles
were assessed for eligibility, and two were excluded for being unrelated to
liver cancer outcomes. Ultimately, nine studies were included in the
qualitative synthesis. This structured screening process ensured methodological
transparency and adherence to PRISMA guidelines.
The geographical distribution of the included
studies (Table 1) indicates a predominance of research conducted in high-income
countries. The United States accounted for the highest number of studies (n =
2). Additionally, one study each originated from the United Kingdom, South
Korea, Spain, Sweden, and Germany. One study was broadly categorized under the
region of Europe and East Asia.
Table 1.
Country
distribution of included studies.
|
Country
Name
|
Total
Count
|
|
United
States
|
2
|
|
Europe
& East Asia
|
1
|
|
United
Kingdom
|
1
|
|
South
Korea
|
1
|
|
Spain
|
1
|
|
Sweden
|
1
|
|
Germany
|
1
|
The summary presented in (Table 2) outlines
the distribution of different study designs included in the dataset. Randomized
controlled trials (RCTs) are the most frequently reported, accounting for three
out of nine studies. Retrospective cohort studies appear twice, while each of
the following designs is represented once: standard cohort study, longitudinal
cohort study with repeated measures, in-vitro experimental study, and
regression analysis. This reflects a diverse mix of research methodologies, with
a slight predominance of experimental approaches, particularly RCTs, which are
widely regarded as the benchmark for evaluating clinical interventions.
Table 2. Methodological
designs of included studies.
|
Study Design
|
Total count
|
|
Randomized
controlled trial
|
3
|
|
Retrospective
cohort study
|
2
|
|
Cohort study
|
1
|
|
Regression
analysis
|
1
|
|
In-vitro
experimental study
|
1
|
|
Longitudinal
cohort study (repeated measures)
|
1
|
Key Characteristics of Included Studies
This table (Table 3) presents the basic
features of each study, providing context for evaluating the study populations,
methodologies, and limitations. These elements help interpret the results and
assess the risk of bias.
Table 3. Key characteristics of studies included in the systematic review.
|
References
|
Country
|
Design
|
Total Participants
|
Age
|
Gender
|
Limitations
|
|
(27)
|
Europe and East Asia
|
Randomized control trial
|
N/A
|
N/A
|
Both male and female
|
MR assumptions, lack of clinical data, limited generalizability
|
|
(33)
|
United Kingdom
|
Longitudinal cohort study
|
2399
|
>35
|
Both male and female
|
Observational design, adherence uncertainty, residual confounding
|
|
(34)
|
U.S.
|
Randomized controlled trial
|
2733
|
60 ± 9.9
|
Both male and female
|
Selection bias, loss to follow-up, confounding
|
|
(35)
|
South Korea
|
Randomized control trial
|
32,692
|
50–60 (avg 58)
|
Comparison
|
Retrospective data, adherence bias, ethnic limitations
|
|
(36)
|
Spain
|
Retrospective cohort study
|
153
|
39–86
|
Majority Child-Pugh
|
Lack of RCTs, pharmacological interactions, genetic variability
|
|
(37)
|
Not Applicable
|
Retrospective cohort study
|
Not applicable
|
Not applicable
|
Comparison
|
Not specified
|
|
(38)
|
United States
|
In vitro experimental study
|
Not applicable
|
Not applicable
|
Not applicable
|
In vitro only, no apoptosis mechanism confirmed
|
|
(39)
|
Sweden
|
Cohort study
|
2104
|
30–94
|
Not applicable
|
Observational design, adherence uncertainty, confounding
|
|
(40)
|
Germany
|
Regression analysis
|
349,210
|
Not applicable
|
Comparison
|
Lifestyle data missing, short follow-up, selection bias
|
Figure 2 displays the risk of bias assessment
for the nine studies included in this review, evaluated across six
methodological domains: participant selection, confounding variables,
measurement of exposure, blinding of outcome assessment, incomplete outcome
data, and selective outcome reporting. Several studies demonstrated a high risk
of bias in multiple domains, particularly in areas such as selective reporting
and participant selection. Some studies showed predominantly unclear risks,
often due to insufficient reporting of study methods. A few studies exhibited
low risk across most domains, particularly in measurement and blinding.
Overall, the figure highlights substantial variation in study quality,
emphasizing the need for cautious interpretation of findings due to
methodological inconsistencies.
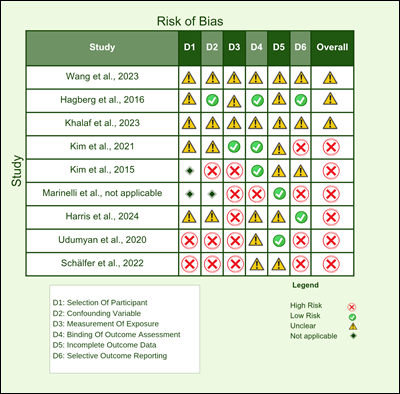
Figure 2. Risk of bias assessment among studies. This figure summarizes the
domain-specific risk of bias for each included study using six criteria:
D1—Selection of Participants, D2—Confounding Variables, D3—Measurement of
Exposure, D4—Blinding of Outcome Assessment, D5—Incomplete Outcome Data, and
D6—Selective Outcome Reporting. Risk levels are indicated by symbols: red (✖) for high risk, green (✔) for low risk, yellow (▲) for unclear risk, and gray diamonds (◆) for not applicable. Overall risk reflects
the cumulative assessment across all domains.
Main Findings of the Studies on the Basis
of Antihypertensive Drugs Classification
This table (Table 4) categorizes the main
findings according to antihypertensive drug classes with serial numbers
referencing specific studies.
Table 4. Main findings of
the studies based on antihypertensive drugs classification.
|
Drug Class
|
Findings
|
References
|
|
Thiazide
Diuretics
|
Associated with decreased HCC risk in both Europeans and East
Asians.
|
(27)
|
|
Beta-Blockers
(BBs)
|
Increased HCC risk in Europeans; No association in UK study;
Reduced liver cancer mortality in Sweden (non-selective BBs better).
|
(27,33,39)
|
|
ACE
Inhibitors (ACEIs)
|
No significant association with HCC risk.
|
(33,40)
|
|
Angiotensin
II Receptor Blockers (ARBs)
|
Reduced recurrence and improved outcomes in HCC patients
following radiofrequency ablation (RFA) (study 5); Increased prostate cancer
risk (study 9).
|
(36,40)
|
|
Renin-Angiotensin
System (RAS) Inhibitors
|
Reduced HCC risk with long-term use in patients with hypertension
and liver disease.
|
(35)
|
|
Diuretics
(General)
|
Associated with increased liver and hematopoietic cancer risks;
decreased prostate and skin cancer risk.
|
(40)
|
|
Calcium
Channel Blockers (CCBs)
|
No significant cancer association.
|
(40)
|
|
Others
(Prazosin, Chlorpromazine)
|
Reduced viability in HCC cell lines; potential cytotoxic effect
in vitro.
|
(38)
|
|
Non-Classified
(e.g., Sorafenib)
|
Adverse hepatic effects not linked to treatment duration or
response.
|
(37)
|
Summary of
Findings (Table 4)
·
Thiazide diuretics demonstrated a
potential protective effect against hepatocellular carcinoma (HCC) in both
European and East Asian populations (27).
·
Beta-blockers demonstrated inconsistent associations with hepatocellular
carcinoma (HCC) outcomes across studies. One study reported an increased risk
of HCC among European users (27), while another conducted in the UK found no
significant protective effect (33). In contrast, a Swedish study observed reduced liver
cancer mortality, particularly with the use of non-selective beta-blockers (39). These conflicting results may be due to differences
in beta-blocker type (selective vs. non-selective), patient populations, study
design, or unmeasured confounding factors.
·
ACE inhibitors and calcium channel
blockers (CCBs) were not significantly associated with liver cancer outcomes in
the reviewed studies (33,40).
·
Angiotensin receptor blockers (ARBs)
provided supportive benefits when used alongside radiofrequency ablation in HCC
management (36,40); however, one
extensive cohort study noted an increased risk of prostate cancer linked to ARB
use (40).
·
Renin-angiotensin system (RAS)
inhibitors, as a broader class, were associated with a decreased risk of HCC
with prolonged use in a South Korean cohort (35).
·
Diuretics, when not categorized into
specific subtypes, were linked with an elevated risk of liver cancer in a large
German regression analysis (40), suggesting outcomes may depend heavily on drug subclass and
patient context.
·
Prazosin and chlorpromazine,
evaluated in vitro, exhibited promising anti-tumor activity, implying potential
for drug repurposing, though clinical evidence is still lacking (38).
·
Sorafenib was associated with
hepatic side effects regardless of treatment duration or response, warranting
caution when used in patients with liver impairment (37).
Influencing Factors in the Relationship Between Antihypertensive Drugs
and Hepatocellular Carcinoma (HCC)
Table below (Table 5) includes influencing factors for liver cancer due
to hypertension management.
Table 5. Influencing factors in the relationship
between antihypertensive drugs and hepatocellular carcinoma (HCC).
|
References
|
Influencing Factors
|
|
(27)
|
Genetic variants, pleiotropy, ethnic differences, hypertension
and liver health, drug metabolism.
|
|
(33)
|
Hypertension, diabetes, liver disease, alcohol use, obesity,
smoking, viral hepatitis, medication adherence, and exposure definition.
|
|
(34)
|
Age, gender, cause of cirrhosis, liver function, comorbidities,
surveillance, and medical management.
|
|
(35)
|
Underlying hypertension and liver disease, medication adherence,
comorbidities (diabetes, obesity), demographics, smoking, alcohol
consumption.
|
|
(36)
|
Tumor characteristics, liver cirrhosis and fibrosis,
comorbidities (renal and cardiovascular health), and pharmacokinetics of
ARBs.
|
|
(37)
|
Pre-existing arterial hypertension (AH).
|
|
(38)
|
Oxidative stress, IC₅₀ concentrations of tested drugs
(chlorpromazine and prazosin).
|
|
(39)
|
Type of Beta-blocker used, liver condition, cancer stage.
|
|
(40)
|
Drug type, comorbidities, large sample size, unaccounted
lifestyle factors, combination therapies.
|
Potential
Effect Modifiers
The studies
reviewed highlighted several categories of factors that may modify the
relationship between antihypertensive medications and hepatocellular carcinoma
(HCC) outcomes:
·
Metabolic and Genetic Factors:
Genetic variability and metabolic differences—including liver-specific drug
metabolism—were noted as possible reasons for differential drug responses
across populations.
·
Comorbid Conditions: Common
coexisting diseases such as hypertension, diabetes, chronic liver disease,
obesity, and cardiovascular disorders were frequently identified as
confounders.
·
Behavioral and Lifestyle Factors:
Alcohol use, smoking, and dietary patterns were recognized as important but
inconsistently measured modifiers of HCC risk.
·
Treatment-Related Factors:
Characteristics such as pharmacokinetics, medication adherence, and the
specific class of antihypertensive drug (e.g., ACE inhibitors, ARBs,
β-blockers, diuretics) were central to observed variations in outcomes.
·
Tumor-Specific Variables: Clinical
features of the tumor—such as size, stage, and vascular invasion—were shown to
influence the impact of antihypertensives when used as adjuncts in HCC therapy.
·
Study and Population Heterogeneity:
Variability in data collection methods, patient demographics, and study design
also contributed to bias and limited comparability across studies.
Discussion
The relationship between antihypertensive
medications and liver cancer remains controversial. While most studies report
no significant association, some have identified links involving common
antihypertensives such as ACE inhibitors (ACEi), angiotensin receptor blockers
(ARB), calcium channel blockers (CCB), and diuretics. Among these, ARBs have
shown a potential protective effect against liver cancer. This is hypothesized
to result from inhibition of the renin-angiotensin system, particularly the
angiotensin II type 1 receptor (AT1R), allowing unopposed stimulation of AT2R,
which may exert anti-proliferative and anti-angiogenic effects (27,41,42).
Experimental models, such as the rat liver
perfusion model, suggest that hyperosmolarity-induced upregulation of the
miR-15/107 family and miR-141-3p may influence liver cell apoptosis and
proliferation (40).
Diuretics have also been associated with
increased liver cancer risk in some studies, with Cox regression analyses
indicating a positive correlation (40).
Additionally, in patients with pre-existing
liver conditions like hepatic steatosis, antihypertensives—particularly CCBs,
ARBs, and ACEis—have been linked to disease progression and elevated liver
cancer risk (43).
This systematic review highlights the nuanced and multifactorial nature
of the association between antihypertensive drugs and HCC. Certain medications,
such as thiazide diuretics, were found to reduce HCC risk in both European and
East Asian populations (27). Similarly, renin-angiotensin system inhibitors—including ACE inhibitors
and ARBs—were linked to improved progression-free survival and lower HCC
incidence in South Korean cohorts (35,36). Evidence from Sweden further suggested that non-selective Beta-blockers
may lower liver cancer mortality (39).
Conversely, some findings raised caution. A German regression analysis
indicated a positive relationship between diuretics and liver cancer risk (40), which contradicts results from a Mendelian randomization study (27). Similarly, Beta-blockers were associated with increased HCC risk in
Europeans (27), while other studies either found no association (33) or reported a protective effect (39). These inconsistencies may stem from differences in drug subtypes,
patient characteristics, and methodological design. Importantly, the
observational design of most included studies (33,36,37,39) limits causal inference and leaves room for residual confounding.
Some studies also examined the repurposing of non-conventional agents.
For instance, in vitro analyses of chlorpromazine and prazosin revealed
cytotoxic effects against liver cancer cells (38), though clinical application remains premature. Additionally, the
adjunctive use of ARBs with radiofrequency ablation was associated with better
outcomes in HCC patients (36), suggesting a potential role in combined treatment strategies.
In summary, while specific antihypertensive drugs—especially RAS
inhibitors and diuretics—may impact HCC risk or progression, findings are
heterogeneous across drug types and populations. These discrepancies likely
reflect variations in underlying liver conditions, genetic backgrounds, drug
mechanisms, and study quality (27,33,35,36,39,40). Carefully designed longitudinal studies are needed to clarify causality
and identify which patient populations may benefit—or be harmed—by specific
antihypertensive therapies in the context of liver cancer.
Recommendations of the Studies
To guide future research and clinical
decision-making, Table 6 summarizes the key recommendations and insights from
each included study, highlighting suggested directions for improving
antihypertensive use in the context of hepatocellular carcinoma (HCC)
prevention and management.
The reviewed evidence collectively underscores the importance of cautious
interpretation regarding the association between antihypertensive medications
and hepatocellular carcinoma (HCC) risk. Several studies (27,35,36,38–40) suggest that specific drug classes—including thiazide diuretics,
Beta-blockers, ARBs, and renin-angiotensin system (RAS) inhibitors—may
influence HCC risk, either positively or negatively. These preliminary
associations highlight the need for further exploration through rigorously
designed prospective studies and randomized controlled trials.
Concurrently, multiple authors (33,34,40) advocate against modifying existing clinical guidelines based solely on
these early findings. Instead, they emphasize maintaining standard cancer
prevention approaches while expanding future research to incorporate broader
risk factors such as genetic predispositions, lifestyle behaviors, and
socioeconomic determinants. Methodological refinements—including the use of
standardized data collection protocols, inclusion of diverse patient
populations, and multi-national study designs—are vital for improving the
reliability and generalizability of future evidence (34).
Table 6. Key recommendations of selected studies.
|
References
|
Recommendations
|
Key
Insights
|
|
(27)
|
Thiazide
diuretics may reduce HCC risk; Beta-blockers may increase it. Consider
individual risk factors and personalize treatment. Further studies are
needed.
|
Potential
drug-specific impact on HCC risk; need for personalized treatment and further
research.
|
|
(33)
|
No protective
effect of ACE inhibitors or Beta-blockers against liver cancer. Continue
using them for cardiovascular indications, not cancer prevention. Emphasize
established preventive strategies.
|
Antihypertensives
not indicated for cancer prevention; focus on established prevention (e.g.,
alcohol reduction, hepatitis management).
|
|
(34)
|
Recommendations
for future research: improve cohort diversity, ensure follow-up, analyze
broader risk factors, and conduct international, standardized studies.
|
Methodological
improvements to enhance validity and generalizability of findings.
|
|
(35)
|
Need for
prospective studies on renin-angiotensin system inhibitors for liver
protection. Encourage tailored approaches in high-risk patients.
|
Possible
protective role of RAS inhibitors; importance of personalized medicine and
longitudinal studies.
|
|
(36)
|
ARBs may be
beneficial in HCC patients undergoing RFA. Recommend further RCTs to confirm
efficacy and safety.
|
Potential
adjunct role for ARBs in HCC treatment; requires validation through RCTs.
|
|
(38)
|
Further studies
on chlorpromazine and prazosin for HCC treatment, including mechanism
exploration. Repurposing may expedite therapeutic development.
|
Drug
repurposing opportunity; need for mechanistic and clinical studies.
|
|
(39)
|
Recommend
further clinical trials on Beta-blockers' survival benefits and their
mechanisms in HCC. Investigate long-term use.
|
Investigate
Beta-blockers' therapeutic potential and long-term outcomes.
|
|
(40)
|
Recommend
future studies on long-term cancer risks of antihypertensives, especially
diuretics and ARBs. Consider lifestyle and genetics. Don’t change clinical
guidelines yet.
|
Cautious
interpretation of current findings; need for comprehensive risk assessment
and further validation.
|
Clinical Implications of the Study
This review underscores the importance of
considering both drug class and patient-specific factors when evaluating the
potential oncologic effects of antihypertensive medications. Among these,
agents targeting the renin-angiotensin system (RAS) may play a particularly
favorable role, especially in individuals with preexisting liver conditions.
Non-selective β-blockers also showed promise in reducing liver cancer
mortality in patients already diagnosed with HCC.
By contrast, diuretics demonstrated inconsistent effects, with some evidence
suggesting benefit while other studies reported increased cancer
risk—emphasizing the need for individualized therapeutic decisions based on
clinical context. Overall, these findings suggest the potential to expand the
therapeutic scope of certain antihypertensive drugs, though careful patient
selection and further validation are essential before clinical application.
Limitations
This review incorporates studies with considerable methodological and
clinical heterogeneity. Included studies range from randomized controlled
trials to observational cohort analyses and in vitro experimental research. A
notable limitation is the frequent reliance on prescription databases rather
than direct confirmation of medication adherence, which may lead to
misclassification of exposure. Additionally, several studies lacked detailed
clinical or lifestyle data, increasing the risk of residual confounding.
Confounding by indication—where the underlying reason for prescribing an
antihypertensive (e.g., cardiovascular disease or cirrhosis) may itself
influence liver cancer risk—was a common challenge in observational studies and
often not adequately controlled for. Selection bias was also a concern,
particularly in studies using region-specific or institution-based cohorts
(e.g., UK-only populations or U.S. veterans), which may not reflect broader
patient demographics. Furthermore, immortal time bias—where patients must
survive a certain period to receive treatment and thus appear to have better
outcomes—may have affected studies lacking clearly defined exposure windows and
time-to-treatment analyses.
Other issues included small sample sizes in certain studies, variations
in follow-up duration, differences in liver disease staging, and inconsistent
classification of antihypertensive drug categories. These limitations
complicate direct comparisons across studies and emphasize the need for
cautious interpretation. Future research should aim to address these biases
through more rigorous study designs, ideally using prospective, longitudinal
data with standardized outcome definitions.
Conclusion
This systematic review highlights the complex and nuanced
associations between antihypertensive drug use and hepatocellular carcinoma. Certain classes—most notably RAS inhibitors and
non-selective β-blockers—emerge as promising candidates for further
exploration in cancer prevention or adjunctive therapy, though definitive
conclusions remain premature. Mixed results for other drug types, such as
diuretics and selective β-blockers, reflect underlying heterogeneity
across study populations, designs, and outcome measures. The evidence,
though promising in parts, is tempered by significant methodological
limitations that prevent definitive conclusions.
Nevertheless, these findings offer a foundation for future research and suggest
a potential role for repurposing certain antihypertensive agents in liver
cancer prevention and treatment. To move the field forward, high-quality,
long-term clinical trials that incorporate diverse populations and standardized
methodologies are essential. These efforts will help determine whether specific
antihypertensive therapies can be safely and effectively integrated into
personalized strategies for patients at risk for or living with HCC.
Author contribution
SN developed the methodology and wrote the methodology
section. SN also conducted data extraction using a predesigned Excel
spreadsheet, capturing key study details, including study design, patient
population, type of antihypertensive medications used, liver cancer outcomes,
and major findings. Additionally, SN oversaw the entire review process
and coordinated the writing of the manuscript. FZ independently verified
50% of the extracted data to ensure accuracy and consistency. FZ also
wrote the results section, contributed to the final review of the manuscript,
played a role in developing the study design, and assisted in refining the
methodology section. AN contributed to refining the search strategy,
participated in the full-text review process, and assisted in synthesizing the
extracted data. AN also built the tables and diagrams for the manuscript
and helped review the methodology section. PD independently conducted
the title and abstract screening using Rayyan software, ensuring the initial
selection of studies. PD also conducted the full-text review for studies
meeting the inclusion criteria and wrote the discussion section. FT
independently verified 50% of the extracted data alongside MA to enhance
data accuracy. FT also contributed to refining the study methodology and
participated in manuscript revisions. MR wrote the introduction section
and assisted in optimizing the search strategy. MR also played a role in
screening full-text articles and contributed to drafting and reviewing the
discussion section. ML independently conducted the title and abstract
screening using Rayyan software, ensuring the initial selection of studies. ML
also wrote the conclusion section and participated in discussions regarding
study inclusion and exclusion criteria. JT contributed to writing the
discussion section and provided critical revisions to improve clarity and
coherence. JT also participated in reviewing the final manuscript to
ensure consistency and accuracy. SS played a role in the quality
assessment of the included studies and assisted in synthesizing the extracted
data. SS also contributed to reviewing the discussion and conclusion
sections to ensure alignment with the study objectives. All authors contributed
to the conception and design of the study, provided input on data
interpretation, and participated in manuscript revisions. All authors approved
the final version before submission.
Funding
There is no funding.
Conflicts of interest
There are no conflicts of interest.
References
Pranali M Wandile MC.
Hypertension and comorbidities: A silent threat to global health. Hypertension
and Comorbidities,;1(1):1-7. 2024 Feb 6;1(1):1–7.
2. Zhou B,
Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective
interventions for elevated blood pressure and hypertension. Nat Rev Cardiol.
2021 Nov 1;18(11):785.
3. Kjeldsen
SE. Hypertension and cardiovascular risk: General aspects. Pharmacol Res. 2018
Mar 1;129:95–9.
4. Zhou B,
Bentham J, Di Cesare M, Bixby H, Danaei G, Cowan MJ, et al. Worldwide trends in
blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based
measurement studies with 19·1 million participants. The Lancet. 2017 Jan
7;389(10064):37–55.
5. Farhadi F,
Aliyari R, Ebrahimi H, Hashemi H, Emamian MH, Fotouhi A. Prevalence of
uncontrolled hypertension and its associated factors in 50–74 years old Iranian
adults: a population-based study. BMC Cardiovasc Disord. 2023 Dec 1;23(1):1–10.
6. Kibone W,
Bongomin F, Okot J, Nansubuga AL, Tentena LA, Nuwamanya EB, et al. High blood
pressure prevalence, awareness, treatment, and blood pressure control among
Ugandans with rheumatic and musculoskeletal disorders. PLoS One. 2023 Aug
1;18(8):e0289546.
7. Oh JH, Jun
DW. The latest global burden of liver cancer: A past and present threat. Clin
Mol Hepatol. 2023 Apr 1;29(2):355.
8. Yao Z, Dai C, Yang J, Xu M, Meng H, Hu X, Lin N. Time-trends in
liver cancer incidence and mortality rates in the U.S. from 1975 to 2017: a
study based on the Surveillance, Epidemiology, and End Results database. J
Gastrointest Oncol. 2023 Feb 28;14(1):312-324.
9. Oster JR,
Materson BJ, Perez-Stable E. Antihypertensive Medications. South Med J. 2023
May 8; 77(5):621–30.
10. Smith SM,
Winterstein AG, Gurka MJ, Walsh MG, Keshwani S, Libby AM, et al. Initial
Antihypertensive Regimens in Newly Treated Patients: Real World Evidence From the OneFlorida+ Clinical Research Network. J Am Heart
Assoc. 2023 Jan 3;12(1):26652.
11. Oster JR,
Materson BJ, Perez-Stable E. Antihypertensive Medications. South Med J. 2023
May 8;77(5):621–30.
12. Chrysant SG, Frohlich ED. Side effects of antihypertensive drugs.
Am Fam Physician. 1974 Jan;9(1):94-101.
13. Smith DK, Lennon RP, Carlsgaard PB. Managing Hypertension Using
Combination Therapy. Am Fam Physician. 2020 Mar 15;101(6):341-349.
14. Shen D, Song S, Hu J, Cai X, Zhu Q, Zhang Y, Ma R, Zhou P, Zhang Z,
Hong J, Li N. The potential of spironolactone to mitigate the risk of
nonalcoholic fatty liver disease in hypertensive populations: evidence from a
cohort study. Eur J Gastroenterol Hepatol. 2025 Sep 1;37(9):1010-1020.
15. Dang C,
Wang R, Shi Y, Liu P, Wang X, Liu J, et al. Genetically proxied therapeutic
effect of antihypertensive drug use, breast cancer, and ovarian cancer’s risk:
a drug-target Mendelian randomization study. BMC Cancer. 2025 Dec 1;25(1):1125.
16. Yang R,
Zhang Y, Liao X, Yao Y, Huang C, Liu L. The Relationship Between
Anti-Hypertensive Drugs and Cancer: Anxiety to be Resolved in Urgent. Front
Pharmacol. 2020 Dec 14;11:610157.
17. Franchi M,
Torrigiani G, Kjeldsen SE, Mancia G, Corrao G. Long-term exposure to
antihypertensive drugs and the risk of cancer occurrence: evidence from a large
population-based study. J Hypertens. 2024 Dec 1;42(12):2107–14.
18. Loosen SH,
Schöler D, Luedde M, Eschrich J, Luedde T, Gremke N, et al. Antihypertensive
Therapy and Incidence of Cancer. J Clin Med. 2022 Nov 1;11(22):6624.
19. Tadic M,
Cuspidi C, Belyavskiy E, Grassi G. Intriguing relationship between
antihypertensive therapy and cancer. Pharmacol Res. 2019 Mar 1; 141:501–11.
20. Wang S, Xie
L, Zhuang J, Qian Y, Zhang G, Quan X, et al. Association between use of
antihypertensive drugs and the risk of cancer: a population-based cohort study
in Shanghai. BMC Cancer. 2023 Dec 1;23(1):425.
21. Watanabe T,
Barker TA, Berk BC. Angiotensin II and the endothelium: Diverse signals and
effects. Hypertension. 2005 Feb 1;45(2):163–9.
22. Zhang S,
Cao M, Hou Z, Gu X, Chen Y, Chen L, et al. Angiotensin-converting enzyme
inhibitors have adverse effects in anti-angiogenesis therapy for hepatocellular
carcinoma. Cancer Lett. 2021 Mar 3;501:147–61.
23. Morris ZS,
Saha S, Magnuson WJ, Morris BA, Borkenhagen JF, Ching A, et al. Increased Tumor
Response to Neoadjuvant Therapy Among Rectal Cancer Patients Taking
Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers.
Cancer. 2016 Aug 15;122(16):2487.
24. Alcocer LA,
Bryce A, De Padua Brasil D, Lara J, Cortes JM, Quesada D, et al. The Pivotal
Role of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor
Blockers in Hypertension Management and Cardiovascular and Renal Protection: A
Critical Appraisal and Comparison of International Guidelines. American Journal
of Cardiovascular Drugs. 2023 Nov 1;23(6):663.
25. Ho CM, Lee
CH, Lee MC, Zhang JF, Wang JY, Hu RH, et al. Comparative effectiveness of
angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers
in chemoprevention of hepatocellular carcinoma: a nationwide high-risk cohort
study. BMC Cancer. 2018 Dec 10;18(1):401.
26. Chen R,
Zhou S, Liu J, Li L, Su L, Li Y, et al. Renin–angiotensin system inhibitors and
risk of hepatocellular carcinoma among patients with hepatitis B virus
infection. Can Med Assoc J. 2024 Aug 12; 196(27):E931–9.
27. Wang Z, Lu
J, Hu J. Association between antihypertensive drugs and hepatocellular
carcinoma: A trans-ancestry and drug-target Mendelian randomization study.
Liver International. 2023 Jun 1;43(6):1320–31.
28. Ma C, Wang
Q, Man Y, Kemmner W. Cardiovascular medications in angiogenesis--how to avoid
the sting in the tail. Int J Cancer. 2012 Sep 15;131(6):1249–59.
29. Qi J,
Bhatti P, Spinelli JJ, Murphy RA. Antihypertensive medications and risk of
colorectal cancer in British Columbia. Front Pharmacol. 2023 Nov 7;14:1301423.
30. Fadil KHA,
Mahmoud EM, El-Ahl SAHS, Abd-Elaal AA, El-Shafaey AAAM, Badr MSEDZ, et al.
Investigation of the effect of the calcium channel blocker, verapamil, on the
parasite burden, inflammatory response and angiogenesis in experimental
Trichinella spiralis infection in mice. Food Waterborne Parasitol. 2022 Mar 1;26:e00144.
31. Cho IJ,
Shin JH, Jung MH, Kang CY, Hwang J, Kwon CH, et al. Antihypertensive Drugs and
the Risk of Cancer: A Nationwide Cohort Study. J Clin Med. 2021 Feb 2; 10(4):771.
32. Yu H, Liu
Z, Wu Y, Zheng L, Wang K, Wu J, et al. Antihypertensive medications and cancer
risk: Evidence from 0.27 million patients with newly diagnosed hypertension.
Front Pharmacol. 2025 Jul 1; 16:1559604.
33. Hagberg KW,
Sahasrabuddhe V V., McGlynn KA, Jick SS. Does Angiotensin-Converting Enzyme
Inhibitor and β-Blocker Use Reduce the Risk of Primary Liver Cancer? A
Case-Control Study Using the U.K. Clinical Practice Research Datalink.
Pharmacotherapy. 2016 Feb 1;36(2):187–95.
34. Kanwal F,
Khaderi S, Singal AG, Marrero JA, Asrani SK, Amos CI, et al. Risk
Stratification Model for Hepatocellular Cancer in Patients With
Cirrhosis. Clin Gastroenterol Hepatol. 2023 Dec 1;21(13):3296-3304.e3.
35. Kim KM, Roh
JH, Lee S, Yoon JH. Do renin-angiotensin system inhibitors reduce risk for
hepatocellular carcinoma?: A nationwide nested
case-control study. Clin Res Hepatol Gastroenterol. 2021 Jul 1;45(4):101510.
36. Facciorusso
A, Abd El Aziz MA, Cincione I, Cea UV, Germini A, Granieri S, et al.
Angiotensin Receptor 1 Blockers Prolong Time to Recurrence after Radiofrequency
Ablation in Hepatocellular Carcinoma patients: A Retrospective Study.
Biomedicines. 2020 Oct 1;8(10):1–13.
37. Miyahara K,
Nouso K, Miyake Y, Nakamura S, Obi S, Amano M, et al. Serum glycan as a
prognostic marker in patients with advanced hepatocellular carcinoma treated
with sorafenib. Hepatology. 2014 Jan 1;59(1):355–6.
38. Harris S,
Nagarajan P, Kim K. The cytotoxic effects of prazosin, chlorpromazine, and
haloperidol on hepatocellular carcinoma and immortalized non-tumor liver cells.
Med Oncol. 2024 Apr 1 ;41(4).
39. Udumyan R,
Montgomery S, Duberg AS, Fang F, Valdimarsdottir U, Ekbom A, et al.
Beta-adrenergic receptor blockers and liver cancer mortality in a national
cohort of hepatocellular carcinoma patients. Scand J Gastroenterol 2020 May 3
[cited 2025 Apr 5];55(5):597–605.
40. Loosen SH,
Schöler D, Luedde M, Eschrich J, Luedde T, Gremke N, et al. Antihypertensive
Therapy and Incidence of Cancer. Journal of Clinical Medicine 2022, Vol 11,
Page 6624. 2022 Nov 8 [cited 2025 Apr 5];11(22):6624.
41. Cho IJ, Shin JH, Jung MH, Kang CY, Hwang J, Kwon CH, Kim W, Kim DH,
Lee CJ, Kang SH, Lee JH, Kim HL, Kim HM, Cho I, Lee HY, Chung WJ, Ihm SH, Kim
KI, Cho EJ, Sohn IS, Park S, Shin J, Ryu SK, Kim JY, Kang SM, Cho MC, Pyun WB,
Sung KC. Antihypertensive Drugs and the Risk of Cancer: A Nationwide Cohort
Study. J Clin Med. 2021 Feb 15;10(4):771.
42. Feng LH,
Sun HC, Zhu XD, Zhang SZ, Li KS, Li XL, Li Y, Tang ZY. Renin-angiotensin
inhibitors were associated with improving outcomes of hepatocellular carcinoma
with primary hypertension after hepatectomy. Ann Transl Med. 2019
Dec;7(23):739.
43. Singh B, Cusick AS, Goyal A, et al. ACE Inhibitors. [Updated 2025
May 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing;
2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430896/

